Details of the Drug
General Information of Drug (ID: DM58VQX)
| Drug Name |
Telavancin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
telavancinum; telavancina; telavancine; 372151-71-8; UNII-XK134822Z0; CHEBI:71229; XK134822Z0; Telavancin [INN]; HSDB 8194; AC1MIWRX; SCHEMBL8287015; CHEMBL507870; DB06402; LS-192933; T-1455; Telavancin, Antibiotic for Culture Media Use Only; Vancomycin, N3''-(2-(decylamino)ethyl)-29-(((phosphonomethyl)amino)methyl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Staphylococcus aureusStreptococcus pyogenesStreptococcus agalactiaeStreptococcus anginosusEnterococcus faecalis
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
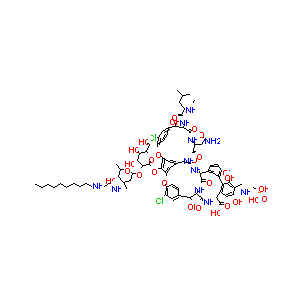 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1755.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 30 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 23 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 31 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Staphylococcus infection | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 1B5Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Telavancin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
